This paper outlines the conversion of a ribozyme to a deoxyribozyme and the activity level of the DNA product before and after several rounds of in vitro evolution. What emerges is that ribozymes and deoxyribozymes do not share inherent catalytic ability based on sequence alone, as the prepared DNA product of the ribozyme sequence showed no catalytic activity initially. Only after 10 rounds of directed evolution did it approach the efficiency of the ribozyme ligase. The author notes "The transfer of function is more difficult because function is an overall property of a macromolecule and cannot be conveyed in a sequential manner." This is reaffirmed when the final, efficient deoxyribozyme is used as template to create a ribozyme. The ribozyme product also showed no catalytic activity initially.
This blog supports the CH795 Special Topics in Chemistry courses taught by Dr. Gavin Williams and Dr. Alex Deiters at North Carolina State University. Please include an illustrative figure when you post a blog entry.
Wednesday, August 31, 2011
Conversion of a Ribozyme to a Deoxyribozyme through In Vitro Evolution
This paper outlines the conversion of a ribozyme to a deoxyribozyme and the activity level of the DNA product before and after several rounds of in vitro evolution. What emerges is that ribozymes and deoxyribozymes do not share inherent catalytic ability based on sequence alone, as the prepared DNA product of the ribozyme sequence showed no catalytic activity initially. Only after 10 rounds of directed evolution did it approach the efficiency of the ribozyme ligase. The author notes "The transfer of function is more difficult because function is an overall property of a macromolecule and cannot be conveyed in a sequential manner." This is reaffirmed when the final, efficient deoxyribozyme is used as template to create a ribozyme. The ribozyme product also showed no catalytic activity initially.
Receptors: Clicking class B GPCR ligands

This article describes research that has been done to design novel ligands for Class B G protein-coupled receptors. The activation and binding domains are fused using 'click chemistry.' An optimized ligand containing unnatural amino acids bound in the nanomolar range and was able to activate its intended receptor with high specificity.
Inhibition of cell proliferation, migration and invasion by DNAzyme targeting MMP-9 in A549 cells
MMP-9 is a enzyme in the family of matrix metalloproteinases. It is believed to be responsible for invasion and metastasis of cancer cells. In this paper the MMP-9 transcript was probed with a complementary DNAzyme. This showed that the DNAzyme lowered the concentration of the MMp-9 transcript, which lowered the rate of invasion and metastasis of the cultured cancer cells.
Rapid, Photoactivatable Turn-On Fluorescent Probes Based on an Intramolecular Photoclick Reaction

This paper shows a new strategy for designing the photoactivatable fluorescent probes: Photoactivatable fluorescent probes are invaluable tools for the study of biological processes with high temporal and spatial resolution. It is reported that the fluorophore is generated in situ through an intramolecular tetrazole-alkene cycloaddition reaction (“photoclick chemistry”). It has been demonstrated that a protein-targeting core is linked to a photoactivatable, alkene-appended tetrazole as a new strategy for designing turn-on fluorescent probes.
Developing Visible Fluorogenic ‘Click-On’ Dyes for Cellular Imaging
 This paper shows how various biomolecules such as nucleic acids and proteins can be fluorescently labeled via 'click' reaction. The benzothiazole alkyne is weakly fluorescent until it is 'clicked' onto an amino acid or nucleoside with an azide group to form the triazole, which greatly increases the fluorescent intensity so that it can be seen in cells. Since the alkyne is weakly fluorescent, the background fluorescence is greatly reduced.
This paper shows how various biomolecules such as nucleic acids and proteins can be fluorescently labeled via 'click' reaction. The benzothiazole alkyne is weakly fluorescent until it is 'clicked' onto an amino acid or nucleoside with an azide group to form the triazole, which greatly increases the fluorescent intensity so that it can be seen in cells. Since the alkyne is weakly fluorescent, the background fluorescence is greatly reduced.
Tuesday, August 30, 2011
Optical Switch for Motor Protein
 Photocleavable small molecules introduce a unique method to control and study biological functions. This article explains the process and advantages of using caging compounds to study biological processes and provides a study in which a motor protein was similarly spatiotemporally controlled. I personally found this article interesting because caging groups are a integral tool for the Deiters lab, here at NC State. Not only does this article highlight how caging technology is becoming more popular but also how biological tools are always expanding.
Photocleavable small molecules introduce a unique method to control and study biological functions. This article explains the process and advantages of using caging compounds to study biological processes and provides a study in which a motor protein was similarly spatiotemporally controlled. I personally found this article interesting because caging groups are a integral tool for the Deiters lab, here at NC State. Not only does this article highlight how caging technology is becoming more popular but also how biological tools are always expanding.
Thin Gold Film-Assisted Fluorescence Spectroscopy for Biomolecule Sensing
The fluorescent spectroscopy technique described in this paper is able to identify specific biomolecule interactions with small molecules in a complex biological environment. By using thin gold film, incident light passes through and excites fluorescent antibody labels which are conjugated on small molecules. I think this could be a method in identifying small molecule-protein interactions and binding affinity simultaneously.
Monday, August 29, 2011
Ribozyme-Catalyzed Transcription of an Active Ribozyme
This work on a RNA polymerase ribozyme is along the lines of the presentation topic today in CH795-06. The authors make use of directed evolution and screen products with a water-in-oil emulsion system called "compartmentalized bead tagging" (CBT). Here, the authors use a recombinant ribozyme to create a general RNA polymerase that can copy many various RNA sequences. By ultimately using it to replicate another ribozyme, they support the viability of the RNA world hypothesis, where RNA constitutes transcript, enzyme, and product.
Sunday, August 28, 2011
Probing essential nucleobase functional groups in aptamers and deoxyribozymes by nucleotide analog interference mapping of DNA
This paper shows a chemical method for structural probing of function within DNA molecules:
"Nucleotide analog interference mapping of DNA (dNAIM) is here introduced as a new non-enzymatic interference-based approach that enables high-throughput identification of essential nucleobase functional groups in DNA aptamers and in the catalytic core of deoxyribozymes. Nucleobase-modified ribonucleotides are statistically incorporated into DNA by solid-phase synthesis, employing the 2’-OH group as a chemical tag for analysis of interference effects."
Friday, August 26, 2011
Loss of CD4 T-cell–dependent tolerance to proteins with modified amino acids
In this paper, unnatural amino acids were used to probe the immune response in mice. Mouse proteins containing unnatural amino acids were injected into mice, causing an immune response to both the modified and native proteins. It was found that CD4 T cells only recognized the mutated protein, but generated antibodies which were able to target both native and mutant proteins. Since the unnatural amino acids used in this study are similar to post-translational modifications induced under conditions of stress, the authors reasoned that the same immune response may occur in those circumstances.
Thursday, August 25, 2011
N-Sulfanylethylanilide Peptide as a Crypto-Thioester Peptide
In this paper, the authors show how they use a N-terminal Sulfanylethylanilide peptide linker to improve Native Chemical Ligation (NCL) process. They reported that this N-Sulfanylethylanilide moiety can efficiently substitute the thioester intermediate usually formed during NCL. They also report the first one-pot, four peptide components coupling under kinetic conditions.
This is quite promising for the synthesis of large proteins.
Ca2+ Regulation of Mitochondrial ATP Synthesis Visualized at the Single Cell Level

Intracellular Ca2+ levels play a crucial role in the control of ATP synthesis. However, the spatiotemporal correlation between ATP and Ca2+ remains unclear due to the inability to visualize these factors within same individual cells. In this paper a new red-shifted ATP probe called GO-ATeams was developed, in which green fluorescent protein (GFP)and orange fluorescent protein (OFP) were used as a FRET pair. This new probe allows for better resolution at the single cell level.
Chemoenzymatic Synthesis of Cryptophycin Anticancer Agents by an Ester Bond-Forming Non-ribosomal Peptide Synthetase Module

Cryptophycins (Crp) are a group of cyanobacterial depsipeptides with activity against drug-resistant tumors. Although they have been shown to be promising, further efforts are required to return these highly potent compounds to the clinic through a new generation of analogues with improved medicinal properties. Herein, we report a chemosynthetic route relying on the multifunctional enzyme CrpD-M2 that incorporates a 2-hydroxy acid moiety (unit D) into Crp analogues. CrpD-M2 is a unique non-ribosomal peptide synthetase (NRPS) module comprised of condensation–adenylation–ketoreduction–thiolation (C-A-KR-T) domains. We interrogated A-domain 2-keto and 2-hydroxy acid activation and loading, and KR domain activity in the presence of NADPH and NADH. The resulting 2-hydroxy acid was elongated with three synthetic Crp chain elongation intermediate analogues through ester bond formation catalyzed by CrpD-M2 C domain. Finally, the enzyme-bound seco-Crp products were macrolactonized by the Crp thioesterase. Analysis of these sequential steps was enabled through LC-FTICR-MS of enzyme-bound intermediates and products. This novel chemoenzymatic synthesis of Crp involves four sequential catalytic steps leading to the incorporation of a 2-hydroxy acid moiety in the final chain elongation intermediate. The presented work constitutes the first example where a NRPS-embedded KR domain is employed for assembly of a fully elaborated natural product, and serves as a proof-of-principle for chemoenzymatic synthesis of new Crp analogues.
Discovery of Kibdelomycin, A Potent New Class of Bacterial Type II Topoisomerase Inhibitor by Chemical-Genetic Profiling in Staphylococcus aureus
This Chemistry and Biology paper explains the use of a high throughput screen to discover a new antibacterial compound. Kibdelomycin was found to inhibit topoisomerases in a wide variety of gram-positive bacteria.
Mutasynthesis – uniting chemistry and genetics for drug discovery
"Mutasynthesis couples the power of chemical synthesis with molecular biology to generate derivatives of medicinally valuable, natural products. Recently, this technique has been exploited by Cambridge-based biotech company Biotica Technology Ltd, and their collaborators, to generate promising new variants of the polyketide anti-cancer compounds rapamycin and borrelidin."
Mutasynthesis is basically precursor directed biosynthesis combined with mutagenesis of the producing strain. In this approach, one synthesizes analogues of an intermediate in a biosynthetic pathway and mutates the producing strain so that it can't provide the natural intermediate. Thus the rest of the pathway is forced to utilize the analogues. Pretty cool.
Mutasynthesis is basically precursor directed biosynthesis combined with mutagenesis of the producing strain. In this approach, one synthesizes analogues of an intermediate in a biosynthetic pathway and mutates the producing strain so that it can't provide the natural intermediate. Thus the rest of the pathway is forced to utilize the analogues. Pretty cool.
Wednesday, August 24, 2011
Mussel protein adhesion depends on interprotein thiol-mediated redox modulation

Tuesday, August 23, 2011
The rise, fall and reinvention of combinatorial chemistry
We talked about small molecule libraries, library assembly, and library diversity in class today. This is a short highlight by Tom Kodadek (Scripps FL) in which he summarizes the most recent developments. Give it a quick read, since some of the discussed technologies will be discussed in class (e.g., DNA-templated synthesis and DNA-encoded libraries).
Expanding the Genetic Code of an Animal
In this short communication Greiss and Chin show that they were able to incorporate UAAs into the nematode worm. They are able to place lysine derivatives in the place of the amber stop codon allowing for the expression on GFP and mCherry. They state that this is the first genetically encoded incorporation of UAAs into a multicellular organism.
Chemical Approaches to Understand the Language of Histone Modifications
Epigenetics is the study of gene expression without the change in DNA sequence. Histones which are proteins wrapped around DNA play an important role in regulating gene activity. This paper talks about various synthetic strategies to site specifically modify histones which regulate the structure and function of the chromatin. Most studies focus on alterations on DNA to control gene regulation however; this paper gives us a wider understanding in gene regulation through histone modifications and epigenetics
A fluorescent turn-on probe for the detection of alkaline phosphatase activity in living cells
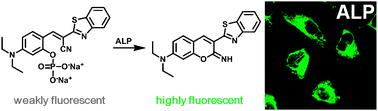
This paper from ChemComm describes the design and characterization of a fluorescent probe that is activated by intracellular alkaline phosphatase activity. It enables the real-time imaging of endogenous alkaline phosphatase (ALP) activity in living cells, and the fast and quantitative analysis of enzyme activity at the single-cell level.
Monday, August 22, 2011
Nanostructures that mimic VEGF as a strategy for ischemic tissue repair

This article was from the latest issue of PNAS that discusses a new strategy for ischemic tissue repair. Nanofibers with a mimetic active site of the protein VEGF self-assemble after the addition of customizable peptide molecules. These nanofibers were shown to be effective at tissue repair, with more than twice the increase in blood vessel density than VEGF. What was interesting to me in this article was that synthetic customizable molecules could mimic a natural protein so well. Not only did this decrease the cost of treatment, but decreased the frequency of injections. With high costs of medical treatments, this strategy seems like a step forward in the right direction.
Subcellular Protein Localization by Using a Genetically Encoded Fluorescent Amino Acid

In the latest issue of ChemBioChem, G. Charbon et al. reported a technique for in-vivo fluorescent labeling of proteins without improper protein assembly and/or function. They successfully placed very small fluorescent tag into the bacterial tubulin, FtsZ. The fluorescent, coumarin-derived amino acid (CouAA, see structure above), is encoded into a given sequence. CouAA is transfered to a growing polypeptide when nonsense amber stop codons UAG are present, and the expression of genes terminated by amber codons is not affected.
Reprogramming bacteria to seek and destroy an herbicide
Here the Gallivan group discusses the selection of a synthetic riboswitch that turns on gene function in response to the herbicide atrazine. Though the concentrations of the herbicide required to activate translation of the gene are high compared to what would be found in nature this is a good first step to solving a real-world problem with the use of riboswitches. The gene used is able to metabolize the harmful herbicide into a non-toxic compound.
Structure-Guided Design of Cell Wall Biosynthesis Inhibitors That Overcome β-Lactam Resistance in Staphylococcus aureus (MRSA)
This article focuses on using alkyl boronic acids as inhibitors of Penicillin-Binding Proteins (PBPs) which can be used aganist β-Lactam resistant pathogens . The authors have identified alkyl boronic acids that are active against methicillin-resistant S. aureus (MRSA). This article explains how the spread of antibiotic resistance by bacterial pathogens is increasing and how there is a need to find the next active family of antimicrobials.
Sunday, August 21, 2011
Restricted Lateral Diffusion of Luteinizing Hormone Receptors in Membrane Microdomains
This Journal of Biological Chemistry paper expands on the prior discovery that hormone receptors aggregate upon binding of hormones. The authors used gold nano-particles to image individual receptors and discover that they are contained to a small portion of the membrane during activation. Point mutation to disturb membrane anchoring was also examined to determine that without membrane anchoring, compartmentalization did not occur.
Saturday, August 20, 2011
Collective synthesis of natural products by means of organocascade catalysis
In this article, Jones et al. took what they knew about natural product biosynthesis and developed a synthetic mimic to make complex molecules. In other words, instead of having to do one reaction, purify the product, and then have it be the starting material for another reaction, the authors are able to just add the starting materials and have the reaction cascade to it's final product. This very similar to the way products are made biologically but eliminates the need for enzyme intermediates.
Friday, August 19, 2011
Structure of the HIV-1 Frameshift Site RNA Bound to a Small Molecule Inhibitor of Viral Replication
This ACS Chemical Biology paper out of U-Wisconsin Madison discusses a small molecule that binds to HIV RNA and inhibits viral replication by blocking the -1 framshift site required for HIV replication. The small molecule was discovered through high-throughput screening. They look at the small molecule binding through thermodynamics and NMR, and use that information to form structural relationships of the RNA:small molecule complex. I found it to be a very interesting method using NMR to determine specific binding characteristics of a small molecule inhibitor of RNA function. The information can assist in designing RNA binding small molecules, and the development of new compounds for HIV treatment.
Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study
In a recent paper in Angewandte, the Wu lab compares different reaction conditions for copper-catalyzed and strain-promoted 'click' reaction for different protein bioconjugation purposes. Overall, the efficiency of the Cu-catalyzed reactions is the highest, if an optimal ligand is used: they recommend BTTA. If you are doing 'click' reactions, I highly recommend taking a look at the conditions and experimental procedures in this paper.
The author, Peng Wu, was a Sharpless graduate student and a Bertozzi postdoc, and thus is an expert in [3+2] bioconjugation reactions.
The author, Peng Wu, was a Sharpless graduate student and a Bertozzi postdoc, and thus is an expert in [3+2] bioconjugation reactions.
An orthosteric inhibitor of the Ras-Sos interaction

Disruption of protein-protein interactions can be accomplished using alpha helical mimics of one of the interacting proteins. This approach has been used to disrupt Ras-Sos interactions which are key for cellular signal transduction pathways. A hydrogen bond surrogate (HBS) approach was used to enhance helicity of the peptide and substitution of non-interacting residues with polar amino acids was used to make the peptide more soluble. In vitro and in vivo assays were performed to determine that the optimized peptidomimetic binds to the Sos binding site of Ras, but keeps GDP from being exchanged for GTP, and therefore keeps the downstream proteins from being activated.
Chemical genetics identify eIF2a kinase heme-regulated inhibitor as an anticancer target.
This is a great article that demonstrates the use of some specific N,N'-diarylureas to activate the heme-regulated inhibitor kinase leading to a decreasing occurrence of elF2-GTP-tRNAiMet translation initiation complex. These results could bring better understanding and potential treatments for diseases such as cancer and certain anemias.
Tuesday, August 16, 2011
Microfluidic technologies for synthetic biology
Chemical biology and biomolecular engineering will play important roles in the growth of the field of synthetic biology. This review covers the latest microfluidic technologies that can provide "dynamic profiling of gene expression/regulation with high resolution, highly sensitive on-chip and off-chip detection of metabolites, and whole-cell analysis" for synthetic biology applications.
Wednesday, August 10, 2011
Directed Evolution by Yeast Surface Display
RNA Parts for Synthetic Biology
Thursday, August 4, 2011
In-Stem-Labeled Molecular Beacons for Distinct Fluorescent Color Readout
Molecular beacons typically contain a fluorophore and a quencher at the 3' and 5' end of the hairpin stem. Here, the authors incorporated a FRET pair internally into a stem. This design led to well-separated emission bands, a high contrast between duplex and hairpin, and enhanced an signal-to-noise ratio. Although not trivial to implement, I am wondering if this could also be applied to the detection of other DNA assembly events, e.g., the hairpin chain reaction.
Wednesday, August 3, 2011
High-Density, Multiplexed Patterning of Cells at Single-Cell Resolution for Tissue Engineering and Other Applications
The DNA encoded 2D and 3D patterning of cells was recently reported in Angewandte Chemie. The authors used the biotinylation of cell surface proteins followed by binding to streptavidin-conjugated oligonucleotides. This and other DNA-cell labeling methodologies had previously been reported. I am surprised they didn't do a cell surface display of streptavidin and used biotinylated DNA. I am also wondering if that could be done in a light-activated fashion...
Monday, August 1, 2011
Chemical Genetics
If you have not read it yet, please take a look at this recent review by David Spring on Chemical Genetics. He is giving a very concise overview of the design of a Chemical Genetics projects and provides references to four recent case studies.
Genetically Encoded 1,2-Aminothiols Facilitate Rapid and Site-Specific Protein Labeling via a Bio-orthogonal Cyanobenzothiazole Condensation
The Chin lab described the genetic encoding of a 1,2-aminothiol chemical handle in bacterial cells. This was achieved through the incorporation of an unnatural lysine amino acid into a protein, using an evolved pyrrolysyl-tRNA synthetase. The 1,2-aminothiol was used for selective bioconjugation reactions in the presence of cysteine residues. Interestingly, the 1,2-aminothiol was linked to the lysine via an amide bond instead of a carbamate bond that is typically more readily accepted by the synthetase.
Subscribe to:
Comments (Atom)